Platinum, a precious and rare metal, boasts a range of remarkable characteristics that make it highly sought after across various industries. Recognized for its exceptional resistance to corrosion and high melting point, platinum’s noble metal status adds to its allure and utility. Its luster and durability also make it a popular choice for jewelry, offering a combination of aesthetic appeal and lasting wear.
In technology and industry, platinum’s role cannot be overstated. It serves as a crucial component in catalytic converters for vehicles, which helps reduce harmful emissions, and as a catalyst in the manufacturing of numerous chemicals. Aside from its industrial significance, platinum holds an esteemed place in the medical field for its use in life-saving devices and cancer treatments, as well as in electronics where it ensures the reliability of computer hard disks and high-precision instruments.
Key Takeaways
- Platinum is valued for its corrosion resistance and high melting point.
- It is indispensable in automotive, chemical, and medical applications.
- The metal’s rarity and noble properties underpin its use in jewelry and electronics.
Properties and Classification
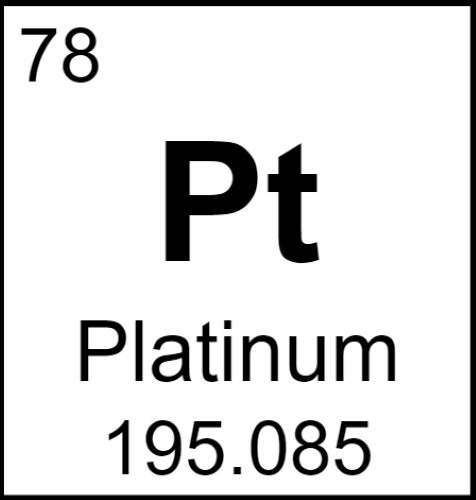
Platinum, with its remarkable resistance to corrosion and high melting point, stands out as a highly valued chemical element in various industries. It is part of the transition metals and falls under the precious metals category due to its scarcity and wide range of applications.
Physical Characteristics
Platinum is a dense, malleable metal that has a significant heft to it. Recognized by its silvery-white color, platinum’s density is approximately 21.45 grams per cubic centimeter. As a metal with a high melting point of around 1,768 degrees Celsius (3,214 degrees Fahrenheit), platinum can withstand extreme conditions, which contributes to its versatility in industrial applications.
Chemical Properties
With an atomic number of 78, platinum is a transition metal characterized by its remarkable chemical stability. This metal is one of the least reactive elements, showing a notable resistance to oxidation and corrosion. Such chemical properties make platinum ideal for various uses, including laboratory equipment and catalytic converters in automobiles.
Sources and Production

Global Reserves
South Africa is the powerhouse in platinum production, boasting the largest reserves globally. Russia and Canada also contribute significantly, with additional notable reserves in countries like Colombia. Platinum deposits are often found alongside other metals, particularly platinum group metals (PGMs) such as palladium and iridium.
- South Africa: Predominant in PGM reserves, particularly platinum.
- Russia: Notable for the Norilsk deposits with PGMs.
- Canada: Home to the Sudbury Basin which is rich in nickel and associated PGMs.
- Colombia: Minor player with emerging potential.
Mining and Refining
Platinum mining involves both open-pit and underground techniques where it is primarily extracted as a byproduct of nickel and copper mining. After mining, the complex refining process begins. It includes crushing, milling, and froth flotation to concentrate the PGMs. The subsequent smelting and chemical processes eventually purify and isolate platinum.
- Extraction Techniques:
- Open-pit mining
- Underground mining
- Byproduct of nickel and copper mining
- Refining Processes:
- Crushing and milling of ore
- Froth flotation
- Smelting
- Chemical refinement for pure platinum
Industrial and Technological Uses

Catalysis in Industry
In the realm of industrial catalysis, platinum serves as an efficient catalyst in various chemical reactions due to its ability to withstand harsh conditions and facilitate reactions without being consumed. It is a key component in catalytic converters used in the automotive industry to reduce harmful emissions by transforming them into less noxious substances. Additionally, platinum catalysts are instrumental in hydrogen fuel cells, where they accelerate the hydrogen oxidation process, playing a crucial role in clean energy technologies.
Electronics and Electrical Applications
Within the electronics sector, platinum is indispensable for its electrical properties. It is used to manufacture electrical contacts and thermocouples because it can endure extreme temperatures without degrading. Its importance extends to electronic components found in a range of devices, aiding in improving their performance and longevity. Platinum’s conductive nature is also vital for emerging technologies, such as fuel cells, that require durable and efficient materials.
Medical and Biomedical Uses
The medical field benefits significantly from the unique properties of platinum. It is an essential material in pacemakers and implantable defibrillators due to its biocompatibility and reliable electrical performance. In chemotherapy, platinum-based drugs are used to treat various cancers, leveraging platinum’s ability to inhibit the division of cancerous cells. This precious metal plays a critical role in developing medical devices and treatments, enhancing the quality of life for many patients.
Jewelry and Investment

Jewelry Crafting
In the world of jewelry crafting, platinum holds a distinguished place. As a rare metal, it embodies exclusivity and sophistication; it is often preferred for creating high-end jewelry like engagement rings and wedding bands. Platinum’s naturally malleable properties enable intricate design possibilities that are resilient to wear and tear. Moreover, its lustrous white sheen offers a more durable and hypoallergenic alternative to white gold. The allure of platinum jewelry rests in its timeless appeal and the lasting legacy it represents when passed down through generations.
Trade and Investment
When it comes to trade and investment, platinum is a sought-after asset. Platinum‘s scarcity makes it a valuable commodity in the market. Investors gravitate towards platinum as a means to diversify their portfolios and hedge against inflation. Often traded in the form of bullion bars or coins, it can be part of a retirement investment strategy or held as a physical asset. Investors appreciate platinum’s tangible and enduring value, recognizing it as a compelling component of a balanced investment approach.
Additional Applications

Automotive Industry
In automotive manufacturing, platinum is a key component used in the production of spark plugs. These spark plugs benefit from platinum’s high melting point and corrosion resistance, ensuring reliable ignition and longevity under the intense conditions of an engine combustion chamber. Moreover, platinum is part of the catalyst exhaust systems in cars that reduce the emission of harmful hydrocarbons, nitrogen oxides, and sulfur dioxide by facilitating chemical reactions that convert these gases into less harmful substances.
Chemical Industry Applications
The chemical industry employs platinum due to its remarkable resistance to corrosion and ability to withstand extreme temperatures. Platinum is involved in producing hydrochloric acid and other strong acids such as aqua regia, where it is used as a catalyst to produce chlorine from salt. This process is crucial for synthesizing a multitude of products, ranging from disinfectants to pharmaceuticals. Platinum’s stable nature allows it to facilitate various chemical reactions without being consumed or contaminating the products.
Frequently Asked Questions
What are the common applications of platinum in the automotive industry?
In the automotive industry, platinum is primarily used in catalytic converters, which reduce harmful emissions from vehicles. It facilitates chemical reactions that convert exhaust gases into less harmful substances.
How is platinum featured in modern electronics and gadgets?
Platinum’s high conductivity and resistance to corrosion make it an excellent material for use in electronic components. It is found in computer hard drives, mobile phones, and other electronic devices.
In what ways is platinum utilized in everyday household items?
Household items often incorporate platinum for its aesthetic and durable nature. It can be seen in glassware production, where platinum is used for its high resistance to heat, and in various home decor items.
Could you explain why platinum is considered more valuable than other metals?
Platinum is considered more valuable due to its rarity, density, and the complex process required to extract and refine it. In addition to these factors, its resistance to wear and tarnish contributes to its high value.
What are the most significant uses of platinum in various industries?
Platinum’s significant uses span from jewelry making, due to its luster and strength, to the medical field where it is used in pacemakers and chemotherapy drugs. Its wide range of applications highlights its industrial importance.
How is the rarity of platinum reflected in its practical uses?
The rarity of platinum is reflected in its use in high-end and critical applications where durability and reliability are paramount. This includes investment, aeronautics, and fine watchmaking, indicating its premium status.








